<< Hide Menu
Danna Esther Gelfand
Jillian Holbrook
Danna Esther Gelfand
Jillian Holbrook
Skills you’ll gain in this topic:
- Identify key elements in biological molecules (C, H, N, O, P, S) and their importance.
- Explain carbon’s unique bonding capabilities and its role in complex molecules.
- Discuss how different molecular structures lead to diverse biological functions.
- Recognize elements essential for building proteins, lipids, and nucleic acids.
- Relate these elements to the formation of cells and tissues.
Matter, atoms, and elements
Organisms, such as ourselves, are made up of matter, which takes up space and has mass. Matter is made up of tiny particles called atoms, which are made up of even smaller particles called protons, neutrons, and electrons.
Atoms can be combined in various ways to form different types of matter, such as solids, liquids, and gases, and to make up different elements, which are substances that cannot be broken down further by chemical reactions. Although there is an entire Periodic Table of Elements, for AP Biology, the essential elements to know are oxygen (O), carbon (C), hydrogen (H), nitrogen (N), calcium (Ca), phosphorus (P), potassium (K), sulfur (S), sodium (Na), chlorine (Cl), and magnesium (Mg).
Compounds are substances that can be broken down further by chemical reactions because they are made of two or more elements that are in a fixed ratio to each other. For example, we learned previously that water, H2O, is made of two hydrogens and one oxygen. Because there is a fixed ratio of hydrogens and oxygen, water is a compound.
Living systems and the organisms in them require constant exchanges of energy and macromolecules. Exchanging matter is what allows an organism to grow and reproduce. By understanding atoms and molecules, we can understand the basis of the elements of life!
Atoms
An element's properties are retained by the smallest unit of mass, called an atom. The subatomic particles that compose atoms are protons (positive charge), neutrons (neutral/no electrical charge), and electrons (negative charge).
The atomic number of an element is determined by the number of protons in the nucleus. In the figure below, the number six is the atomic number of carbon. The atomic mass number is the sum of the protons and neutrons in the nucleus. The atomic mass number from the figure is 12.011.
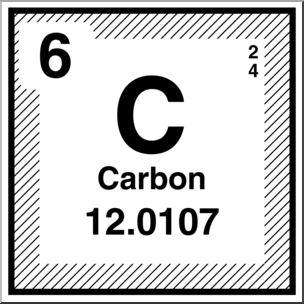
Image courtesy of ABCTeach.
Isotopes
Isotopes are two atoms of an element that have a different number of neutrons. Radioactive isotopes are used for processes that include fossil dating and medical imaging. They decay spontaneously and release energy. An example of a radioactive isotope that is used for dating sites, fossils, and artifacts is Carbon-14, or radiocarbon, which has an atomic nucleus containing six protons and eight neutrons.
Electron Shells
An electron's potential energy (location/structure) is called an energy level or electron shell. When electrons absorb energy, they move up or jump an energy level farther away from the nucleus. When electrons release energy, they move closer to the nucleus.
Elemental Building Blocks
Carbon
Carbon is the building block of the major macromolecules/organic molecules: carbohydrates, lipids, proteins, and nucleic acids. It is a major component of compounds and helps form cells in organisms.
Why is carbon such an important biological element? Carbon has the unique ability to form four covalent bonds, which is known as tetra-valence. The goal for all atoms is to be stable, and carbon is a stable element that readily bonds with a variety of other elements. Carbon must find four more electrons to fill its outer shell, giving a total of eight electrons to satisfy the octet rule. The octet rule states that atoms will lose, gain or share electrons to achieve an electron configuration of eight valence electrons. (e.g CH4 methane)
Nitrogen
Nitrogen is a building block in proteins, nucleic acids, amino acids, and enzymes. These molecules play crucial roles in many biological processes, including metabolism, cell division, and DNA replication. Nitrogen is even a component of many hormones, such as adrenaline and insulin.
In addition to its role in the synthesis of biological molecules, nitrogen is also important in the environment. Nitrogen is a key element in the nitrogen cycle, which plays a crucial role in the balance of nutrients in ecosystems. While nitrogen is primarily in the atmosphere as a gas, plants and some microorganisms can convert atmospheric nitrogen into a usable form for other organisms through nitrogen fixation, which is essential for the overall functioning of ecosystems.
Phosphorus
Phosphorus is also a useful element in biology because it is a key component of nucleic acids, certain proteins, and lipids. Beyond its role in DNA and RNA, which are essential components of the genetic material in all living organisms, phosphorus is also involved in biological processes like energy production. It also plays a crucial role in the balance of nutrients in ecosystems.
Functional Groups
Functional Groups are accessory elements that give molecules a different structure, therefore, a different function. They can be classified as hydrophobic or hydrophilic based on their charge and polarity characteristics.
- Hydroxyl Group: Hydrogen bonded to Oxygen (OH) attached to the carbon skeleton. (alcohols such as methanol, polar)
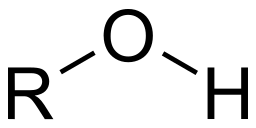
Image courtesy of WikiMedia Commons.
- Carbonyl Group: Double bond (sharing of two pairs of valence electrons) between carbon and oxygen. If the carbonyl group is on the end of the carbon skeleton, it is called an aldehyde. If not, then it is a ketone. (polar)
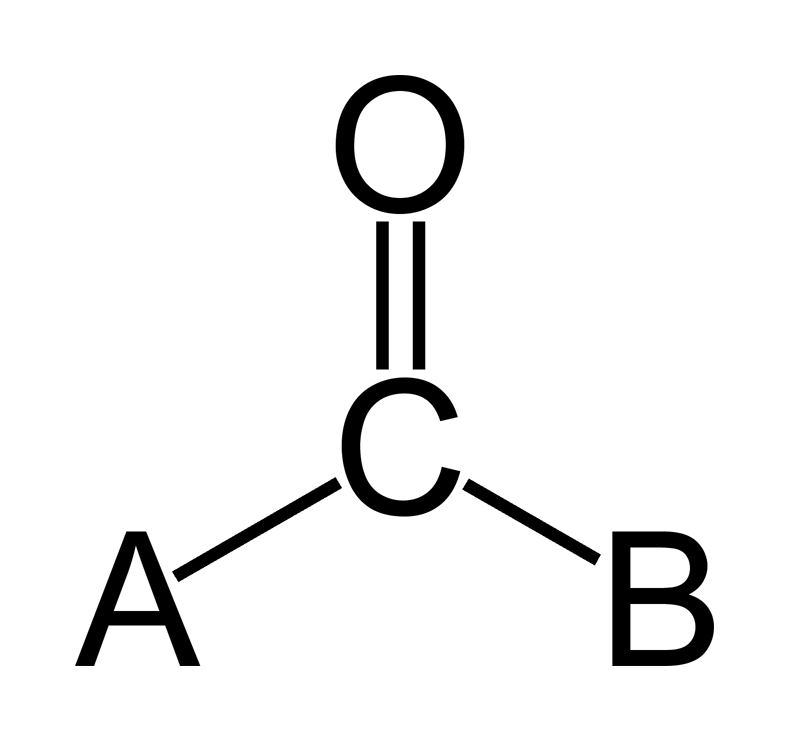
Image courtesy of WikiMedia Commons.
- Carboxyl Group: a combination of carbonyl and hydroxyl where carbon is double-bonded to an oxygen and a hydroxyl. (release H+ into solutions, acidic)
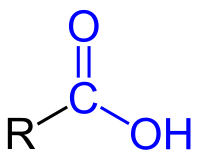
Image courtesy of WikiMedia Commons.
- Amino Group: Nitrogen bonded to two hydrogens and one carbon atom. Amines are organic molecules that have an amino group. (remove H+ from solutions; therefore, basic) Nitrogen is used to build proteins and nucleic acids.
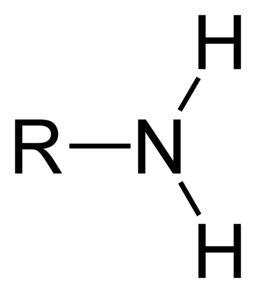
Image courtesy of WikiMedia Commons.
- Phosphate Group: phosphate ion covalently attached to the carbon skeleton. (Lots of energy is used to make nucleic acids and phospholipids; acidic because they release H+ into solutions)
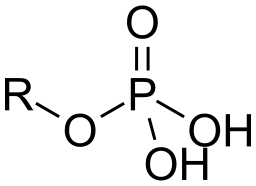
Image courtesy of WikiMedia Commons.
- Sulfhydryl Group: Sulfur bonded to a hydrogen atom. (Polar)
(In the images above, the R represents an unknown part of the molecule that the functional group is attached to)

© 2024 Fiveable Inc. All rights reserved.