<< Hide Menu
Haseung Jun
Tejas Bhartiya
Haseung Jun
Tejas Bhartiya
Skills you’ll gain in this topic:
- Describe how tonicity affects water movement in hypertonic, hypotonic, and isotonic solutions.
- Explain why osmoregulation is essential for water balance in cells.
- Predict cell behavior in different solution types (e.g., swelling, shrinking).
- Relate tonicity to the health and function of cells and organisms.
- Connect osmoregulation to survival strategies in various environments.
Tonicity & Osmoregulation
The movement of water inside and out of the cell is essential to it's survival. Water, just like all other substances, travels from a higher concentration of itself to a lower concentration of itself. This movement can have large impacts on the cell.
Depending on the amount of material outside of a cell compared to inside, the environment outside of a cell can be hypotonic, hypertonic or isotonic to the internal environment of a cell.
A hypotonic solution is one that has LESS solute than the inside of the cell. In this case, water will move to where there is MORE solute (and therefore less water!). Water will move into the cell, causing the cell to expand. think HYPO- HIPPO🦛
A hypertonic solution is one that has MORE solute in it than there is inside of the cell. In this case, water will still move to where there is MORE solute. Water will, therefore, move out of the cell, causing the cell to shrink. think: when you’re HYPER you run outside 🏃♀️An isotonic solution is one that has EQUAL solute in it to that of the cell. In this case, water moves equally in and out of the cell, with no net movement. The cell does not change shape. 💧 Water will attempt to move from an area of high concentration to an area of low concentration until there are equal amounts of water on both sides of the membrane. This is called osmosis. Osmosis allows organisms to control their internal solute composition and water potential.
Below are examples of hypertonic, isotonic, and hypotonic solutions for both a blood cell and a plant cell. As you can see, plant cells are more able to exist in a hypotonic environment due to their cell wall.
Animal Cell:
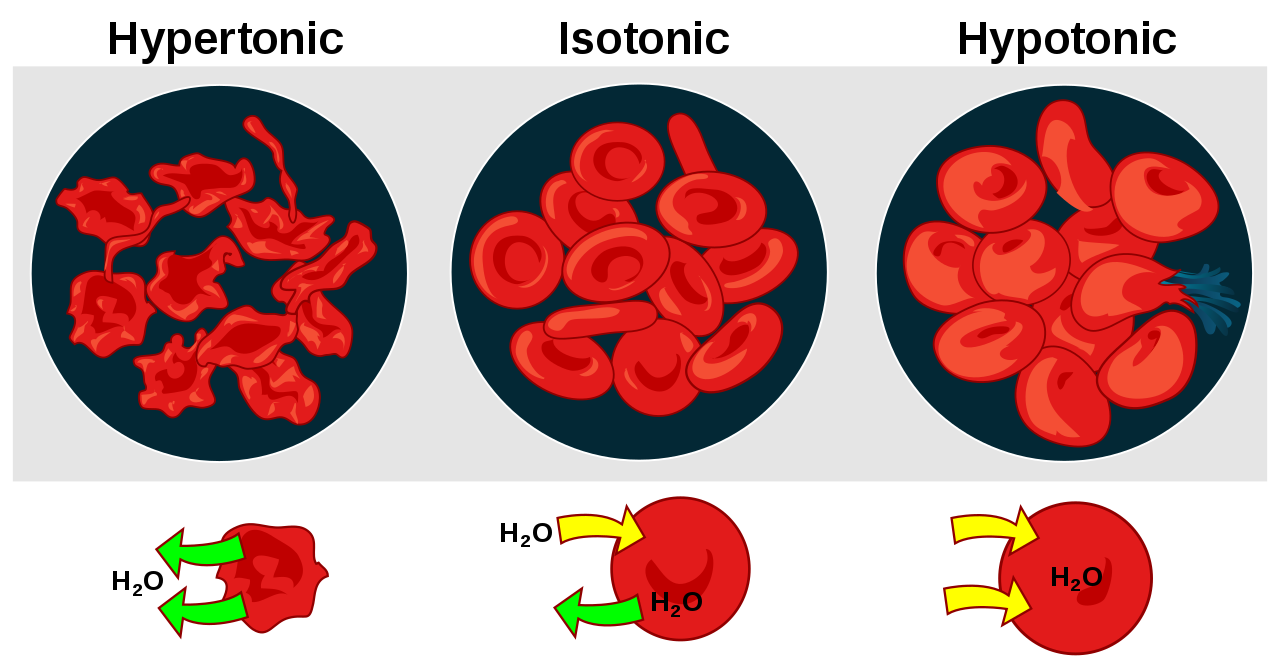
Image courtesy of Wikipedia
Plant Cell:

Image courtesy of Wikipedia
Osmosis
Osmosis is the process of diffusing water across the membrane. Water will always want to move to a more concentrated area in an effort to make the concentrations of inside and outside the cell equal. This is in accordance of homeostasis (trying to keep things normal and counter changes), which is a big deal for AP Bio! So if you ever encounter an FRQ about examples of homeostasis, you know osmosis is one of them!
So when we say water moves down the concentration gradient, we're basically saying water moves to the side that has more "stuff". Water will move from a more watery side to the more concentrated side, meaning water will move from where there's less solute to more solute.
This is because water can move across the membrane relatively easily with the help of aquaporins. However, things like sugar can't cross the membrane easily. But the cell is not happy with uneven concentrations, leaving it up to water to move instead of the "stuff" moving.
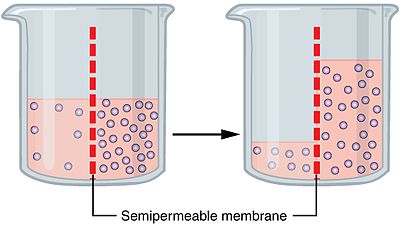
Image Courtesy of Wikipedia
Plants have an easier way to protect themselves from osmotic changes because of its cell wall. The cell wall stays the same size because it's rigid, but the cell membrane can shrink depending on how much water there is inside the cell. So when water comes back into the cell, the cell membrane can expand and squeeze tightly against the cell wall.
Water Potential
Water potential is one of the few math topics AP Biology covers. Basically, it describes osmosis and the direction of the flow of water in mathematical terms. The equation can be found on your equation sheet.

Image Courtesy of College Board
Water in the context of water potential flows from high potential to low potential. The solute potential is the solute concentration in consideration of the water flow. If you add more solute, the water potential of the solution will be lowered, making it likely that more water flows into the solution to counter it.
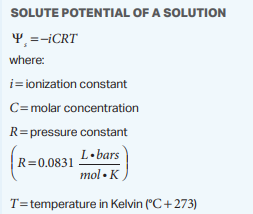
Image Courtesy of College Board
This solute potential is found with the equation shown above.
- The ionization constant refers to the number of ions when the solute dissolves in water. For example, NaCl splits up into Na+ and Cl- when it dissolves in water, making the ionization constant 2. On the contrary, sugar, C6H12O6, doesn't split up into any ions when it dissolves in water, so its ionization constant is 1.
- The molar concentration is how many moles of the solute there are in a given volume of water, with units mol/L.
- The pressure constant is essentially just a number you plug in, no need to do work here 😉
- The temperature of the solute in Kelvin. This is easily found by adding 273 to the Celsius temperature. Almost always temperature will be given in Celsius, so don't worry too much about the Fahrenheit to Celsius conversion.

© 2024 Fiveable Inc. All rights reserved.