<< Hide Menu
Unit 1 Overview: Atomic Structure and Properties
10 min read•june 18, 2024
Jeremy Kiggundu
Dalia Savy
Jeremy Kiggundu
Dalia Savy
AP Chemistry Unit 1: Atomic Structure and Properties
Welcome to Chemistry! If you're like me, you're probably thinking about your life decisions, but do not fret! This study guide is here to help you navigate the triumphs and defeats, the epic highs and lows of AP Chemistry. Unit 1 will cover chemistry basics, including terms like a mole, Avogadro's number, atomic structures, and so much more.
In this unit, you'll learn about how matter is formed and how chemists use specific units to measure amounts of matter, along with calculations involving unit conversions in stoichiometry. This unit develops the foundation for the rest of your chemistry career, so even though we describe it as "the basics," that doesn't make it any less important. In fact, all of these topics appear nearly every day in chemistry. Let's jump right in!

GIF courtesy of @rivedaledit on Tumblr
Without a doubt, Chemistry is the most applicable science there is, which therefore makes AP Chemistry the most lucrative science class you could take while in high school!
Ahem Don’t tell AP Bio kids that I said that🤫.
When taking an AP class, try to keep in mind how much each unit weighs on the examination. In AP Chemistry, Unit 1 accounts for 7-9% of the material you will be tested on.
Introduction to Chemistry ⚗️
In the universe, anything that takes up space and has mass is matter. As we'll discuss below, all matter is built from atoms. Unit 1 will cover some major types of matter, such as molecules, compounds, elements, and pure substances, which all have distinct definitions and compare to each other in different ways. Understanding the different types of matter will help you better understand how matter works in the world.
1.1 Moles and Molar Mass ⚛️
Unit 1 is titled "Atomic Structure and Properties." As such, the unit surrounds one central object: the atom. An atom is the smallest subdivision of an element that maintains the properties of that element. The atom is the primary object in chemistry; it makes up everything around you. Atoms make up molecules and compounds that can react with each other to form other compounds.
You'll learn what makes up an atom and a little bit of quantum mechanics to understand where exactly these things are. The main parts of an atom are the proton, the neutron, and the electron, with the most complicated being electrons. Different models of the atom have shown these three things differently, but today, chemists use quantum mechanics to define orbitals which you'll learn more about moving forward.
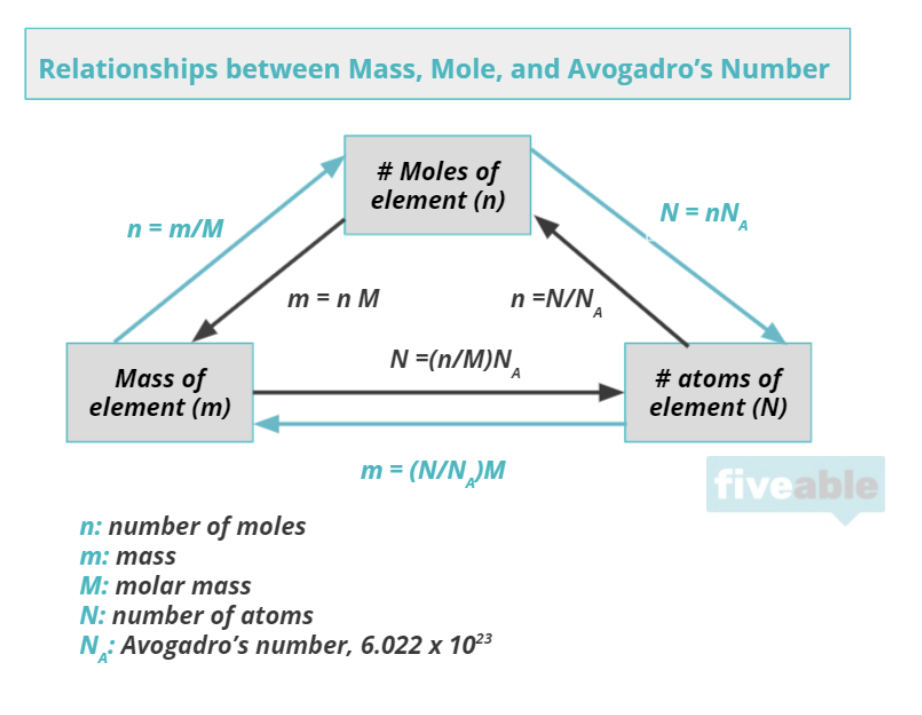
When chemists discuss matter, they do so in terms of moles. Moles help describe the amount of matter (note that this is different from mass, but there is a connection). Chemists measure the number of atoms in a mass of a substance by using the mole. A mole of any given element or compound is 6.022 * 10^23 atoms/molecules. This number is known as Avogadro's number and if you think about it, it is a very large quantity.
The conversion factor from moles to grams and vice versa is known as molar mass and is seen in the units g/mol (or the reciprocal mol/g). Unit 1 focuses intimately on dimensional analysis, commonly known as "unit conversions." Most chemistry problems focus on converting between units, so practicing this skill early on is super important.
1.2 Mass Spectroscopy of Elements 📊
In this section of unit one, we start to break down some of the periodic table. We take a look at an element's symbol, atomic number, and atomic mass. These values are based on the makeup of these elements on an atomic level, which is why it is important to understand the number of protons, neutrons, and electrons in an atom.
When calculating the molar mass of an element, chemists use mass spectroscopy, which is a technique that measures different isotopes for their relative abundance, and then uses a weighted average to find the average atomic mass.
An element does not exist in one form on Earth, so isotopes are variants of an element. You've probably heard of carbon-12, carbon-13, and carbon-14. Although they are all carbon atoms, they have different numbers of neutrons. This technique allows us to see how much of each variant exists on Earth. 🌎
1.3 Elemental Composition of Pure Substances 🧊
This section is where we really talk about matter and how it can be classified according to its state or its composition! You'll be introduced to the basic states of matter: solids, liquids, and gases, though you've probably learned this in earlier classes. We'll talk about the characteristics of each, and then move into a discussion of matter according to its composition.
Pure substances are those that are composed of a single type of atom or molecule. Their composition does not vary from sample to sample. Elements, such as gold and iron, and compounds, such as water and salt, are examples of pure substances.
We will also touch on the law of definite proportions, one of the three fundamental laws that led to the development of the current atomic theory. It states that the ratio of the constituent elements in any pure chemical compound is always the same.
1.4 Composition of Mixtures 🥗
Pure substances and mixtures are the first division in the classification of matter by composition. Where a pure substance is composed of a single type of atom or molecule, a mixture is made up of two or more elements or compounds.
The difference between a homogeneous and heterogeneous mixture is essential to understanding how chemical mixtures can be separated. The components of a homogeneous mixture cannot visibly be seen, but they can in a heterogeneous mixture!
In your AP Chemistry class, you'll most likely become familiar with the laboratory techniques of distillation, filtration, and chromatography. We'll go over the purpose of each and some examples of when they would be used.
1.5 Atomic Structure and Electron Configurations 💭
Here is where unit one pivots from a discussion of matter and mass to one of atomic structure!
When discussing atoms, we also spend time discussing what an atom actually is. As mentioned above, there are three main parts to an atom: protons, positively charged particles, neutrons, neutrally charged particles, and electrons, negatively charged particles.
The protons and neutrons make up the nucleus, whereas electrons orbit around the nucleus. The primary question of quantum mechanics in this unit is "where are the electrons"? You'll learn about electron orbitals which attempt to define zones where electrons probably are. For specific atoms, electrons have certain configurations dependent on quantum numbers that determine their orbital and position.
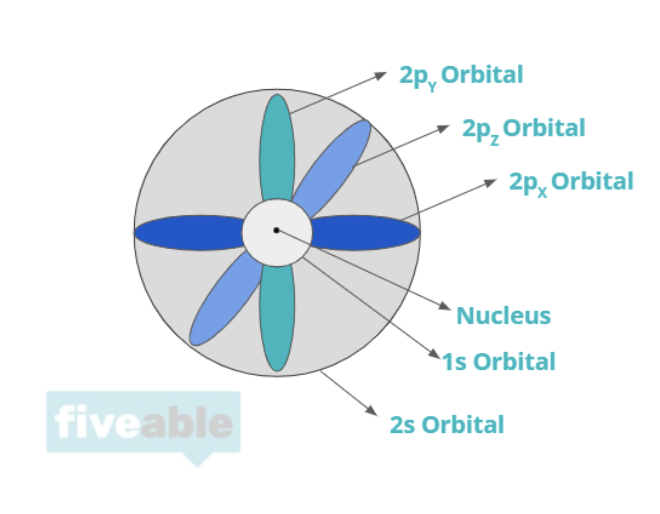
1.6 Photoelectron Spectroscopy 🌈
You can see electron configurations of atoms using PES, photoelectron spectroscopy, which shows an abundance of electrons at certain energy levels. The College Board loves to test whether or not you can read a PES spectrum, so make sure to pay attention to this! We'll go over some multiple-choice and free-response questions that test your knowledge about PES.
1.7 Periodic Trends 📈
The atomic structure can also help us define trends across the periodic table. You'll cover the trends for electronegativity, electron affinity, ionization energy, and atomic/ionic radius. These can be justified by thinking about effective nuclear charge, which is the force experienced by an electron in an orbital.
1.8 Valence Electrons and Ionic Compounds 🔬
Electrons can be categorized into inner core electrons and outer valence electrons. There are certain properties of valence electrons that you should be familiar with in order to understand how different elements bond together.
There are two main categories of bonds: ionic bonds and covalent bonds. Ionic bonds involve a transfer of electrons to form a positive and negative ion held together by Coulombic attraction. Covalent bonds, on the other hand, involve the sharing of electrons. d
AP Chemistry Unit 1 Key Vocabulary
QUICK TIP: You will typically not be asked about the definition of these terms directly. You rather will be expected to apply your knowledge of these terms and justify your claims with evidence. For example, rather than memorizing what ionization energy is, you should be able to understand its trend on the periodic table and explain why the trend exists that way.
Moles - a unit of measurement in chemistry that represents the amount of a substance, which is approximately 6.022 x 10^23 entities.
Molar mass - the mass of a substance in grams that is equal to the number of moles of the substance.
Periodic table - a chart that organizes elements by their atomic number, electron configuration, and chemical properties.
Avogadro's number - 1 mole is equal to 6.022x10^23 atoms of a pure substance.
Dimensional analysis - a method of analysis that is used to convert units of measurement from one system to another.
Atoms - the smallest subdivision of an element that maintains the properties of that element.
Mass spectroscopy - a technique that is used to determine the molecular mass and structure of a compound.
Isotopes - variants of an element due to a difference in the number of neutrons in the nucleus.
Average atomic mass - the weighted average of the atomic masses of all naturally occurring isotopes of an element.
Solid - state of matter characterized by a fixed shape and volume and is held together by strong forces.
Liquid - state of matter characterized by a fixed volume, but a variable shape, and is held together by forces weaker than a solid that allows particles to flow and move freely.
Gas - state of matter characterized by a variable shape and volume and held together by the weakest forces.
Formula units - the simplest whole-number ratio of the atoms or ions present in a chemical compound.
Law of definite proportions - a chemical compound always contains the same elements in the same proportions by mass.
Empirical formula - the simplest whole-number ratio of the atoms present in the compound.
Homogeneous - a mixture in which the constituents are uniformly distributed and cannot be easily separated.
Heterogeneous - a mixture in which the components are not evenly distributed and can be easily separated by physical means.
Distillation - a separation technique that is based on the different boiling points of the components of a mixture.
Filtration - a separation technique that is used to separate a mixture of solid particles and a liquid by passing the mixture through a filter.
Chromatography - a separation technique that is used to separate and purify a mixture of compounds based on their polarity.
Polarity - a measure of the separation of charge within a molecule.
Electron configuration - the arrangement of electrons in an atom or molecule.
Dalton's theory - a model of the atomic structure of matter with four different parts (which we'll discuss further in this unit).
Coulomb's law - a fundamental law that discusses the attraction or repulsion between two charged particles.
Bohr model - a simple model of the atom that proposes electrons are arranged in discrete energy levels or orbits.
Aufbau principle - a rule that is used to predict the electron configuration of an atom, and states that electrons occupy the lowest-energy orbitals available to them.
Pauli exclusion principle - a principle of quantum mechanics that states that no two electrons in an atom can have the same spin.
Hund's rule - a principle in quantum mechanics that states that electrons occupy orbitals of the same energy level in such a way as to maximize the number of unpaired electrons.
Valence electrons - the outermost electrons in an atom that are responsible for the chemical properties of the element.
Core electrons - the innermost electrons in an atom.
Photoelectric effect - the emission of electrons from a metal surface when it is illuminated with light of sufficient energy.
Ionization energy - the energy required to remove an electron from an atom.
Atomic radius - the distance between the center of the nucleus to the outermost valence electron in an atom.
Nuclear charge - a measure of the attractive force that the nucleus exerts on the electrons in the atom.
Electron-electron repulsion - the force of repulsion between two electrons that arises due to their negative electric charges.
Ionic radius - the distance between the center of the ion and the outermost valence electrons in the ion.
Electronegativity - a measure of an atom's ability to attract electrons in a chemical bond.
Electron affinity - the energy change associated with adding an electron to a neutral atom in its gaseous state.
Metals - elements that are characterized by their metallic luster, high electrical and thermal conductivity, and ability to form positive ions by losing electrons.
Nonmetals - elements that are characterized by their lack of metallic properties, such as high electrical and thermal conductivity.
Metalloids - elements that have properties of both metals and nonmetals.
Ionic bonds - bonds formed by the transfer of electrons from one atom to another.
Polar covalent bonds - bonds where electrons are unequally shared between two different elements.
Nonpolar covalent bonds - bonds where electrons are equally shared between two elements with similar electronegativities.
Cation - an atom that loses an electron and gains a positive charge.
Anion - an atom that gains an electron and gains a negative charge.

© 2024 Fiveable Inc. All rights reserved.