<< Hide Menu
Dalia Savy
Kanya Shah
Dalia Savy
Kanya Shah
We're back to solutions! Remember that solutions are homogeneous mixtures where the particles are evenly mixed and the solute is uniformly distributed within the solvent. The solute is the substance that is dissolved, while the solvent is the substance that does the dissolving.
We discussed how to calculate the concentration of a solute dissolved in a solvent, but what does this value depend on? Let's go over solubility!
What is Solubility?
To put it simply, solubility is the ability of a substance to dissolve in a solvent to form a homogeneous mixture. A substance that is soluble in a solvent will dissolve completely to form a solution, while a substance that is insoluble in a solvent will not dissolve and will remain in a separate phase.
The solubility of one substance in another depends on:
- The tendency of systems to become more random (by becoming more dispersed in space)
- The relative intermolecular solute-solute energies compared with solute-solvent interactions. Polar and ionic solutes tend to dissolve in polar solvents, and non-polar solutes tend to dissolve in non-polar solvents (remember “like dissolves like”). This all goes back to intermolecular forces: substances with similar intermolecular forces tend to be miscible or soluble in one another!
👉 Make sure to review intermolecular forces as they will constantly come back in this course!
There are a few solubility rules that will be helpful on the AP Exam and we will return back to these when we discuss precipitation reactions in unit four:
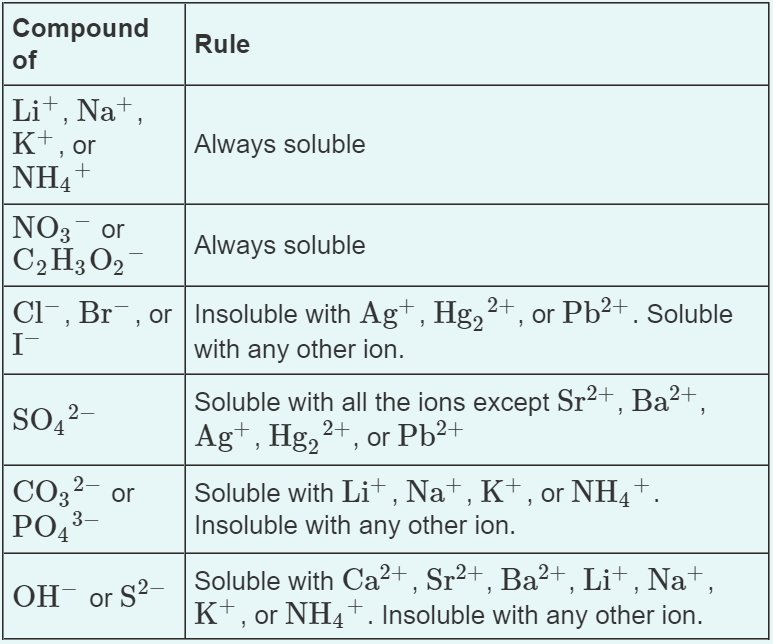
Image Courtesy of Quizlet
You can easily notice that many of the same ions (notably Ag, Hg2 2+, and Pb2+) are often exceptions to these solubility rules. However, do not try to memorize these completely! These, like things like polyatomic ions, are memorized implicitly through usage! That's why by the end of AP Chemistry, most students don't think about charts like these, they simply know them through doing a bajillion problems.
Saturation of Solutions
Every solution, no matter the solute and solvent, has something called a saturation point. Essentially, this is the point at which no more solute can be dissolved in the solvent.
It depends on three things: the temperature, the solvent, and the solute. At a higher temperature, more solute can be dissolved (for the majority of solutes). This can be seen in the following solubility curve:
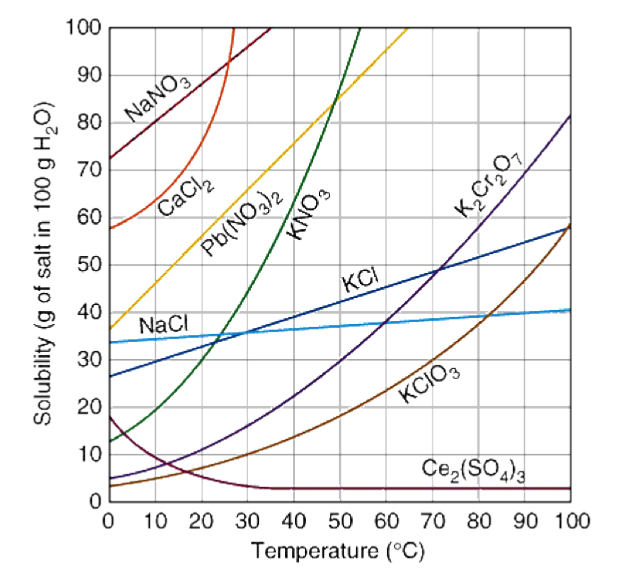
Image Courtesy of Dynamic Science
Solubility curves are graphs that show the relationship between the solubility of a substance in a solvent and the temperature of that solvent. These curves are especially helpful when predicting the solubility of a substance at different temperatures.
When learning about a new type of graph, you always want to note the axes:
- The x-axis is the temperature of the solvent in degrees Celcius.
- The y-axis is the mass of the substance that is dissolved in a given amount of solvent.
The solubility of a substance is usually expressed in grams per 100 mL of solvent, which we can see in the graph above
Take a look at the dark blue curve that represents the solubility of potassium chloride (KCl). At approximately 70 degrees Celcius, there will be almost 50 grams of KCl dissolved in the solution. This value specifically forms a saturated solution.
** Practice reading these graphs and ensuring you understand what information can be obtained from them, as well as what relationship is shown.**
Saturated Solutions
A saturated solution is a solution in which the maximum amount of solute has been dissolved in a solvent at a specific temperature. You can also think about solubility as the amount of solute needed to form a saturated solution at any particular temperature.
Once you have reached the saturation point, any additional solute (at that temperature) will fall out of the solution and not dissolve. Essentially, with saturated solutions, you are on the solubility curve. ⚖️
Undersaturated Solutions
Undersaturated solutions are what their name implies - a solution that has not yet reached saturation. ⬇️
With an undersaturated solution, you can add more solute and it will continue to dissolve (with the temperature remaining constant). Like saturated solutions, an undersaturated solution can be modeled using a solubility curve, representing a point below the solubility curve.
Supersaturated Solutions
Supersaturated solutions are a type of solution where you have more solute than you can dissolve in the solvent. You have basically surpassed the saturation point. ⬆️
In order to accomplish this, a chemist heats up the solution and then adds enough solute to saturate it at that temperature. The chemist would then slowly cool the solution down to the temperature they desire. This creates a supersaturated solution that when agitated will produce crystals. These crystals are extra solutes coming out of the solution, allowing the supersaturated solution to return to its saturated state.
With this being said, you can identify when a solution will be supersaturated by looking above the solubility curve on a graph.

A supersaturated solution of CH3COONa, GIF Courtesy of Gyfcat
Factors Affecting Solubility
The solubility of a substance is influenced by several factors, including the temperature of the solvent, the concentration of the solvent, and the presence of other substances.
Polarity
As mentioned before, the polarity of both the solvent and solutes has a huge effect on solubility. This concept can always be thought of as "like dissolves like." Nonpolar substances are more likely to dissolve in nonpolar solvents and polar substances are more likely to dissolve in polar solvents.
Pressure
Pressure only affects the solubility of gases. At higher pressures, more gas can be dissolved in the solvent, while at lower pressures, less gas will dissolve. In other words, as pressure increases, solubility increases as well. This phenomenon explains why soda🥤 becomes flat over time.
This can also be seen using Henry's Law: C = kP, where...
- C stands for the concentration of the dissolved gas in molarity
- k stands for Henry's law constant
- P stands for the partial pressure of the gas above the solution This equation is just an extra piece of information that your teacher may speak about, and it is not necessary for the AP.
For solids and liquids, pressure does not affect solubility. This is very important and you will have to start paying attention to the phase at which the compounds you are discussing are in.
Temperature
As mentioned before when taking a look at solubility curves, as temperature increases, the solubility of solids and liquids increases as well. This is due to the increased kinetic energy of the solvent molecules at higher temperatures, allowing them to more effectively dissolve the solute. Remember, temperature can be thought of as the average kinetic energy of particles.
This trend is different for gases: as temperature increases, the solubility of the gas decreases.
👉This will be discussed in greater depth when we go over unit seven later in this course.
Concentration of the Solvent
The solubility of a substance generally increases as the concentration of the solvent increases. This is due to the increased number of solvent molecules present, which allows for more solute to dissolve.
Presence of Other Substances
The solubility of a substance can be influenced by the presence of other substances, such as electrolytes or solutes. The presence of these substances can affect the solubility of the solute by altering the chemical nature of the solvent or by competing for the same binding sites on the solvent molecules.
Surface Area
The solubility of a substance is generally influenced by the surface area of the solute. A smaller solute particle size will generally increase the solubility of the substance, as it allows for a greater surface area to be exposed to the solvent.

© 2024 Fiveable Inc. All rights reserved.