<< Hide Menu
Hope Arnett
Dalia Savy
Hope Arnett
Dalia Savy
What Is Stoichiometry Useful For?
In the previous section, we looked at qualitatively analyzing chemical reactions. Now, we’ll learn how to quantitatively analyze reactions using stoichiometry! It may seem like a lot of math at first, but once you do more practice, you’ll be more confident and become a stoichiometry master 👨🏫
Key Concepts to Understanding Stoichiometry
In earlier units, you learned about moles, molar mass/molar volume, molarity, and Avogadro's number. Stoichiometry is all about using mole ratios and these measurements and manipulating them to get to our desired unit. First, let's recap some key concepts to understand in stoichiometry:
- Balanced chemical equation: A balanced chemical equation is a written representation of a chemical reaction that shows the reactants on the left side and the products on the right side, with the number of atoms of each element balanced on both sides of the equation.
- Mole: The mole is a unit of measurement in chemistry that represents the amount of a substance. One mole of a substance is equal to Avogadro's number, which is 6.022 x 10^23 atoms or molecules.
- Stoichiometric coefficients: The coefficients in a balanced chemical equation represent the relative amounts of the reactants and products in a chemical reaction. The coefficients can be used to calculate the amount of reactants and products that are required or produced in a chemical reaction.
- Stoichiometric calculations: Stoichiometric calculations involve using the balanced chemical equation and the mole concept to predict the amount of reactants and products that are required or produced in a chemical reaction. These calculations are based on the principle of the conservation of mass, which states that the total mass of the reactants in a chemical reaction is equal to the total mass of the products. As you've probably noticed, stoichiometry is used specifically to quantify the amount of reactants and products used in a chemical reaction. Therefore, it becomes especially helpful in the laboratory and becomes second nature to chemists.
Mole Ratios
A mole ratio is a ratio of the amounts of two or more substances in a chemical reaction, expressed in moles. Without a mole ratio, it'd be difficult to use stoichiometry to quantify values in the laboratory. It’s important to comfortably know the relationship between certain units as we do mole ratios. Below is a list of these measurements:
- At STP, one mole of an ideal gas fills 22.4 L of volume. This is also known as molar volume and this value can be found on the AP Chemistry reference sheet.
- As discussed above, one mole = 6.022 x 10^23 particles. Avogadro's number is also on the AP Chemistry reference sheet.
- One mole is represented by the molar mass (the number of grams there are in a mole). Each element's molar mass is on the periodic table. If we take a look at hydrogen, there are 1.008 grams in one mole. You can find mole ratios by looking at a chemical reaction🧪. In the reaction below, 1 mole of C2H5OH (ethanol) and 1 mole of oxygen gas are needed to produce 2 moles of carbon dioxide and 3 moles of water. A sample mole ratio would be 1 mole of O2 to 2 moles of CO2. Keep in mind that 2 moles of CO2 to 1 mole of O2 would mean the same thing even though this may seem backward.
Practice Stoichiometry Problems
Question 1
How many moles of potassium metal is required to fully react with 11.6 moles of water?
Step 1
Write the chemical reaction, if it’s not given. Be sure to balance it to satisfy the law of conservation of mass. 2K (s) + 2H₂O (l) → 2KOH (aq) + H₂ (g)
Step 2
Identify the known measurement. In this case, we’re given the value of 11.6 moles of water💧.
Step 3
Since our given value is already in moles, we just need to write the appropriate mole ratio. We want a ratio that cancels❌ out the known measurement units (moles of H2O) and brings in the unit we want (moles of K).
Looking at the chemical equation, for every 2 moles of H2O, we need 2 moles of K metal.
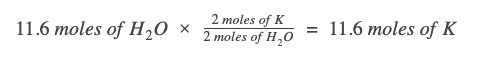
** Note: Writing out the units and the molecule the value belongs to in the dimensional analysis will help you exponentially. Please do it!**
One way to make sure you’re writing the correct mole ratio is to keep track of the units in your work. Since we need the unit "moles of K," we have to ensure that every other unit gets canceled out:

Question 2
If you have 105.2 g of ethanol (C₂H₅OH), what is the maximum volume of carbon dioxide that can form at STP?
Step 1
Write the chemical equation and balance it: C₂H₅OH (l) + 3O₂ (g) → 2CO₂ (g) + 3H₂O (l)
Step 2
Identify the known measurement: 105.2 g of ethanol.
Step 3
Since our given value is in grams, we need to convert it to moles. To do this, we will calculate the molar mass (g/mol) of C₂H₅OH using the molar mass of each element found on the periodic table: 2(12.01g) + 6(1.008g) + 16.00g = 46.07 g/mol
Using this value, we can write a mole ratio to get rid of the unit of grams of ethanol.
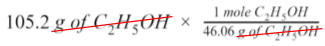
Step 4
Uh oh!😩 We don’t want moles of C₂H₅OH--we want the volume of carbon dioxide produced. Now that we have moles, though, we can go over to CO₂ with a mole ratio. Looking at the balanced chemical equation, for every 1 mole of C₂H₅OH, we can make up to 2 moles of CO₂.
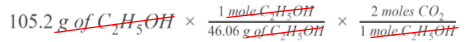
Step 5
Almost there🎉! We have a unit of CO₂, but we want its volume. Since this reaction occurs at STP, we can use the value of the molar volume at STP.

Good work! With 105.2g of ethanol, we could make 102. L of CO₂😮.
You will see stoichiometry used with the ideal gas law (PV=nRT) and molarity (moles/mass or moles/volume, often denoted as M). Check out the practice problems to see how you’ll see them in problems.
🎥 Watch AP Chemistry teacher Mónica Gracida teach the basic concepts of stoichiometry.
General Steps
Here is a neat list of the general steps for tackling stoichiometry problems:
- Write out the balanced chemical equation, if not given.
- Identify the known measurements given to you.
- If the known measurement is in grams, convert it to moles.
- Multiply by a mole ratio using the coefficients in the chemical equation.
- Repeat steps 3-4 as many times as necessary.
- Cross out your units and circle the final one to make sure you set it up correctly!
Try on your Own!
The following reaction occurs at STP:
How many particles of BrF will be produced with 160.0g of Br2?
Answer
Step 1 includes using the molar mass of Br2 to convert to moles of Br2. Step 2 is the mole ratio of Br2 to BrF using their respective stoichiometric coefficients. Step 3 uses Avogadro's number to convert the number of moles to the number of particles of BrF.

© 2024 Fiveable Inc. All rights reserved.