<< Hide Menu
Dalia Savy
Jacob Jeffries
Dalia Savy
Jacob Jeffries
From the College Board
Develop Your Understanding of this unit
According to the College Board, "Unit 4 focused on chemical changes💥; in Unit 5 students will develop an understanding of the rates at which chemical changes occur and the factors that influence the rates📈. Those factors include the concentration of reactants, temperature, catalysts, and other environmental factors. Chemical changes are represented by chemical reactions, and the rates of chemical reactions are determined by the details of the molecular collisions.
Rates of change in chemical reactions are observable and measurable. When measuring rates of change, students are measuring the concentration of reactant or product species as a function of time. These chemical processes may be observed in a variety of ways and often involve changes in energy as well. In subsequent units, students will describe the role of energy⚡ in changes in matter."
Big Idea Questions
- Why are some reactions faster than other reactions?
- How long will a marble statue last? 🗿
- How can a sports drink cure a headache? 🤕
- Why does bread rise? 🍞
AP Chemistry Unit 5: Kinetics
Kinetics is the study of the rate of chemical reactions, essentially how fast a chemical reaction goes. While we can study whether a reaction will actually happen or not using many tools, such as solubility rules and activity series, one cannot use these same tools to study the specific rate at which a reaction will happen. This is analogous to knowing whether someone moved or not since the last 5 seconds have passed, versus knowing with which speed they moved in that time interval.
About 7-9% of the exam tests your knowledge from this unit. ✍️
5.1 Reaction Rates
The rate of a reaction is simply how quickly a reaction proceeds and produces products. As a reaction progresses, the concentration of the reactants decreases as they are used to create products. The change in concentration over a certain period of time is how we measure the "speed" of a reaction.
The rate of reaction can be written mathematically as Rate = -Δ[Reactant]/t or as Rate = Δ[Product]/t. The units for rate are mol/Ls, also notated as Ms⁻¹ or mol L⁻¹ s⁻¹. You may also see that seconds will change to hours, etc. The units of the rate of reaction are something to keep track of.
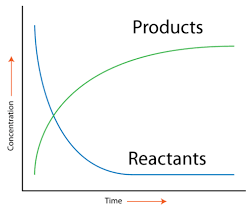
Image Courtesy of CK-12
Graphically, rate is the slope of the line between two points on either curve. This is because the slope of a line represents the change in concentration over the change in t, which as we discussed, gives us the rate!
In this study guide, we'll also discuss several physical attributes that can influence the rate of a reaction, including concentration, temperature, surface area, pressure, and the presence of a catalyst.
5.2 Introduction to Rate Law
Once you have a solid idea of what the rate of reaction is, it's time to learn how to quantitatively determine the rate. We do this by using a rate law.
In chemistry, a rate law is an equation that describes the relationship between the rate of a chemical reaction and the concentrations of the reactants. A rate law is defined by saying: R = k[A]ⁿ[B]ᵐ... where:
- R is the rate of the reaction
- k is the rate constant
- [A] and [B] represent concentrations of the reactants, and
- n and m are reaction orders for each reactant Reaction order describes how the rate of the reaction changes as the concentration of each reactant changes. It is specific to the reactant, which is why a reaction can be described with respect to an individual reactant.
The rate constant, k, is a tricky thing to understand. Essentially, it serves as a proportionality constant for the reaction to take place. We'll go into this deeper, but the biggest thing you have to know is that k is temperature specific. The value of k depends entirely on the temperature the reaction is taking place at.
5.3 Concentration Changes Over Time
Now that we've got the basics down, we can move forward into integrated rate laws. But wait, didn't we just go over rate laws? The rate law described in the last section is really called a differential rate law, and it describes how the rate of a chemical reaction changes with respect to the concentration of reactants or products.
On the other hand, an integrated rate law describes how the concentration of a reactant or product changes with respect to time during a chemical reaction. The integrated rate law is obtained by solving the differential rate law for the concentration, and it is usually written in the form of an equation that describes the concentration as a function of time.
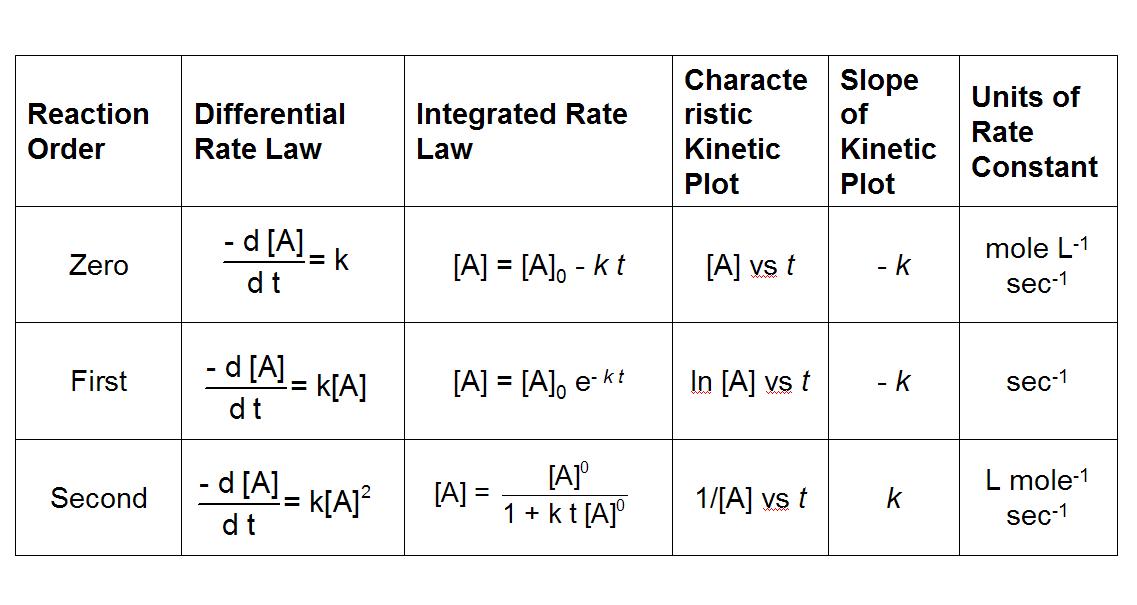
Image Courtesy of Chem FSU
In this study guide, we go over the integrated rate laws for zeroth, first, and second-order reactions. For the AP, it is important to understand how to use these integrated rate law equations and what they look like on a graph.
5.4 Elementary Reactions
Unfortunately, the only true way that rate laws can be found is through an experiment. It's a common mistake of chemistry students to look at the stoichiometric coefficients of the reactants and use those as reaction orders, but you cannot do that!
The reaction 2A → B is not necessarily second order, it could be, but we won't know until we run an experiment. The experiment run to find a rate law is quite simple. All it involves is running multiple trials of a reaction with different concentrations of each reactant, typically doubling one, and then seeing how the rate reacts to this change in concentration. These reactions must be run at the same temperature since k is temperature dependant.
5.5 Collision Model
We've got some of the math down, but what is kinetics conceptually? Remember that kinetics is the study of the rate of chemical reactions, or basically, how fast a chemical reaction can go. In this study guide, you'll learn that in order for a reaction to proceed, or be successful, very specific conditions must be satisfied.
The collision model is used to describe how chemical reactions occur. In order for a reaction to occur, the reactant molecules have to collide with enough energy and in the correct orientation for a chemical bond to be formed or broken.
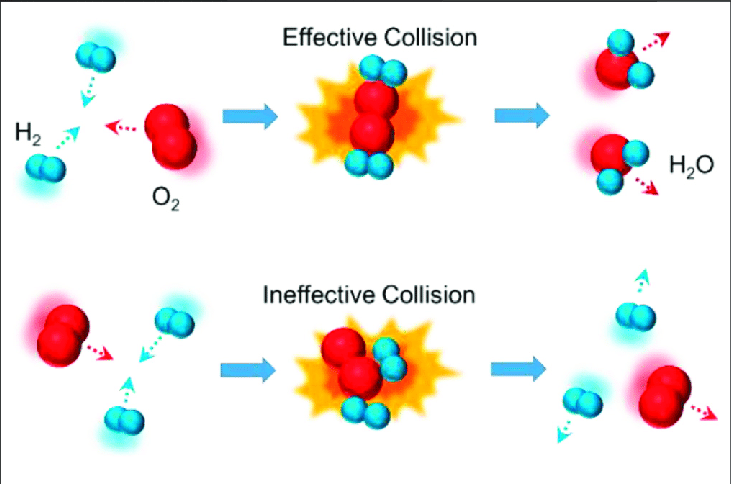
Image Courtesy of Research Gate
There's one big caveat to the collision model, and that's that not every collision will result in a chemical reaction. Reactant molecules will collide, but not all of them will result in an effective collision that satisfies the two conditions of the collision model. The rate of a chemical reaction is thus determined by the frequency of collisions between reactant molecules, as well as the probability that a collision will lead to a successful reaction.
5.6 Reaction Energy Profile
How much energy is "enough" for an effective collision to occur? In this study guide, we'll focus on the reaction coordinate, or potential energy diagram, of a reaction. With these graphs, we can see the changes in energy occurring as the reaction progresses.
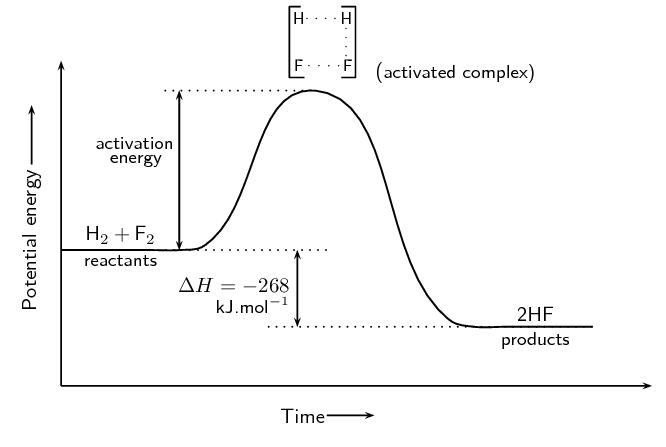
Later in this unit, we will break down every piece of this graph, but let's focus in on activation energy. Activation energy is the energy required to break the bonds in a reaction to go from the reactants to the activated complex to the products. You can think of activation energy as the minimum amount of energy required to start a chemical reaction!
5.7 Introduction to Reaction Mechanisms
The order of a reaction must either be determined experimentally, as we discussed, or through analyzing a reaction mechanism, which deeper describes the chemical details of a reaction. Instead of analyzing the reactions macroscopically, this method allows chemists to see a relatively more accurate picture regarding how and why a chemical reaction happens, instead of simply analyzing what products come from what reactants.
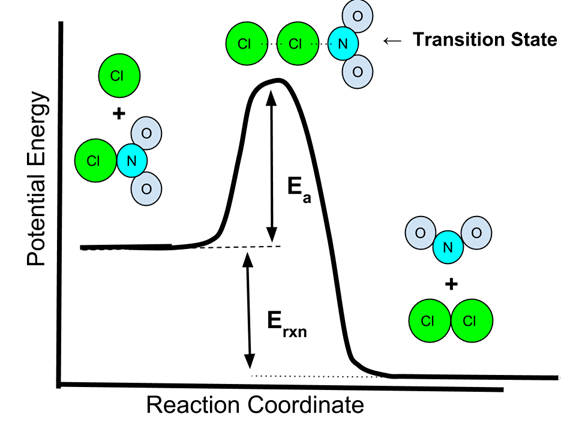
Image Courtesy of SoftSchools
These reaction mechanisms motivate finding overall rates, as the rates of each mechanistic step can be used to find the overall rate of the reaction by analyzing something called the rate-limiting step, which is conceptually similar to the concept of limiting reactants in stoichiometry.
5.8 Reaction Mechanism and Rate Law
By breaking down a reaction mechanism, we can identify substances called intermediates and catalysts.
Intermediates are species that are formed during a chemical reaction, but are not the final products. They are short-lived species that play a role in the mechanism of the reaction and are often crucial in determining the rate and outcome of the reaction.
Catalysts, on the other hand, are substances that change the rate of a chemical reaction without being consumed in the process. They work by providing an alternative pathway for the reaction to occur, often with a lower activation energy.
In this study guide, a free-response question from the 2019 AP Chemistry exam is broken down to show you how a kinetics question has been asked and what you can do to ace it.
5.9 Steady-State Approximation
The steady-state approximation is a method used in chemical kinetics to simplify the mathematical analysis of a reaction by assuming that the concentration of a certain reactant or intermediate remains constant over time, even though it is continuously being consumed and formed. This allows the rate of the reaction to be calculated using a simplified rate law and can be useful in cases where the intermediate species has a very short lifetime or when the reaction is proceeding at a very slow rate. The validity of the steady-state approximation depends on the reaction conditions and the system being studied, and it may not always provide accurate results.
5.10 Multistep Reaction Energy Profile
A multistep reaction energy profile is a graphical representation of the energy changes that occur during a chemical reaction that involves multiple steps. It typically includes the activation energies for each step, the transition state, and the overall energy change for the reaction. It also shows how the reactant molecules progress through the different steps of the reaction to form the products. The energy profile can help to understand the rate and mechanism of the reaction, as well as to predict and optimize the reaction conditions. It can also give an insight into the thermodynamic and kinetic stability of the intermediate species formed during the reaction.
This section is basically a more complex version of key topic 5.6. 🙃
5.11 Catalysis
As we discussed earlier, catalysts lower the activation energy for a reaction and increase the rate of reaction. A catalyst can be a chemical substance or an enzyme that is added to the reaction mixture, or it can be a natural component of the reaction mixture.
📝Unit 5 Key Vocabulary
Kinetics - the branch of chemistry that deals with the study of the rate of chemical reactions and the factors that affect it.
Rate of reaction - the change in the concentration of a reactant or product per unit time.
Average rate of reaction - the change in the concentration of a reactant or product over a given period of time.
Instantaneous rate of reaction - the rate of a chemical reaction at a specific moment in time.
Rate law - an equation that describes the relationship between the rate of a chemical reaction and the concentrations of the reactants.
Rate constant - a value that is obtained from the rate law equation, that describes how fast a chemical reaction occurs.
Reaction order - the exponent of the concentration term in the rate law equation.
Integrated rate laws - mathematical expressions that describe the relationship between the concentration of a reactant or product and time during a chemical reaction.
Half-life - the time it takes for the concentration of a reactant or product to decrease by half during a chemical reaction.
Stoichiometric coefficients - the numbers in front of the chemical formulas in a balanced chemical equation that indicate the relative amounts of reactants and products.
Elementary reaction - a chemical reaction that occurs in a single step.
Collision model - a theoretical framework used to describe chemical reactions, based on the idea that chemical reactions occur when reactant molecules collide with enough energy and in the correct orientation.
Effective collision - a collision between reactant molecules that has sufficient energy and the correct orientation to lead to a chemical reaction.
Activated complex - a short-lived intermediate species that forms during a chemical reaction and is the transition state between reactants and products.
Activation energy - the minimum energy required for a chemical reaction to occur.
Arrhenius equation - an equation that describes the relationship between the rate constant of a chemical reaction and the temperature.
Elementary steps - the individual steps in a chemical reaction mechanism.
Rate-determining step - the step in a chemical reaction mechanism that controls the overall rate of the reaction.
Reaction mechanism - the sequence of elementary steps that describes how a chemical reaction occurs.
Intermediate - species that are formed during a chemical reaction, but are not the final products.
Catalyst - a substance that increases the rate of a chemical reaction without being consumed or changed in the process.

© 2024 Fiveable Inc. All rights reserved.