<< Hide Menu
8.2 pH and pOH of Strong Acids and Bases
3 min read•june 18, 2024
Dylan Black
Jillian Holbrook
Dylan Black
Jillian Holbrook
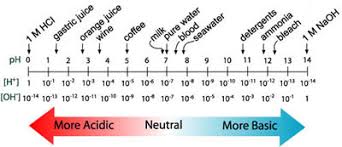
pH and pOH Explained
pH and pOH are extremely important topics in Unit 8 and AP Chemistry as a whole. So then, what is pH? pH is simply a measure of the concentration of protons in a solution.
pH
Essentially, pH is measured by the concentration of H+ ions, meaning it measures how acidic a solution is. A higher pH means a lower concentration of H+, implying a more basic solution and vice versa (I know, that is really annoying). So, we get why there is an H in pH - H means hydrogen, and pH measures the concentration of hydrogen ions. What about the p? In chemistry, 'p' is a symbol that means negative log. p(anything) = -log(anything). Thus, pH = -log([H+]).
For example, if we had a solution that had 0.01M H+, that would mean pH = -log(1 * 10^-2) = -(-2) = 2 (note that log() implies log base 10).
pOH
If pH = -log([H+]), then one can logically find that pOH = -log([OH-]) and is, in essence, the opposite of pH. Where pH is the concentration of H+, pOH is the concentration of OH-. pOH measures how basic a solution is. A low pOH means a highly basic solution and vice versa.
The Autoionization of Water
To elaborate, let us suggest we had the reaction OH- + H+ --> H2O, which can be described as the protonation of OH- or the autoionization of water. The aforementioned reaction serves as the net ionic for a strong acid-strong base interaction. This reaction can further be described using equilibrium by flipping the reaction to say that H2O ⇌ OH- + H+. The K value for this reaction is a constant called Kw. Kw = 1 * 10^-14.
Think about this reaction a little bit. From Kw that we were given before, we can write that Kw = [OH-][H+]. Therefore, 10^-14 = [OH-][H+]. Now, we can take the negative log of both sides:
14 = -log([OH-][H+]) = -log([OH-]) + -log([H+])
From here, it is clear then that pH + pOH = 14.
pH/pOH of Strong Acid and Strong Base Solutions
Finding the pH of Strong Acid/Base Solutions
For these examples, we will just be looking at pH since it is the most common measure of acidity, but remember, pOH can be easily calculated from this. When dealing with strong acids (and by extension strong bases), what is important is that these reactions go fully forwards. That is to say, they have a K value so high that to call it an equilibrium is negligible. Therefore, all of the acid/base will dissociate.
Take a look at the following example:
Suppose we have a 1M solution of HCl. The dissolution of HCl looks like: HCl --> H+ + Cl- (we're gonna stick with the Arrhenius definition of acids to make things simpler). Because this reaction goes completely forward, we know that 1M of HCl will dissolve into 1M of H+ and 1M of Cl-. To find pH, we take the -log([H+]).
pH = -log(1) = -0 = 0. Therefore a 1M solution of HCl has a pH of 0.
The same idea applies to strong bases. Suppose we had 1M NaOH. NaOH --> Na+ + OH-, so pOH = -log([OH-]) = -log(1) = 0.
To find pH, we then plug our value into pH + pOH = 14, which tells us that the pH of a 1M solution of NaOH is 14.
The List of Strong Acids
There are seven strong acids you need to memorize for AP Chemistry. Luckily, they're not that bad, and they become second nature after a while through practice:

These acids completely ionize in aqueous solutions to produce hydronium ions. As such, the concentration of H3O+ in a strong acid solution is equal to the initial concentration of the strong acid, and thus the pH of the strong acid solution is easily calculated.

© 2024 Fiveable Inc. All rights reserved.