<< Hide Menu
Unit 9 Review: Applications of Thermodynamics
4 min read•june 18, 2024
Dylan Black
Jillian Holbrook
Dylan Black
Jillian Holbrook
Welcome to the final unit of AP Chemistry!! Over the last eight units, you battled stoichiometry, chemical reactions, quantum mechanics, intermolecular forces, acids and bases, kinetics, equilibrium, and so much more. There’s just one last beast waiting for you to conquer: thermodynamics. Thermodynamics was introduced originally in Unit 6 with the idea of thermochemistry. You learned about concepts like energy, heat, and enthalpy and their connections to chemical reactions. These ideas will be expanded upon in Unit 9.
Introduction to Thermodynamics
As you may have learned in Unit 6, thermodynamics is the study of energy in chemical reactions. The prefix thermo- might make you predict that we will be discussing a lot about temperature changes, but, in fact, temperature is a pretty small part of thermodynamics!
Instead, we will spend most of our time talking about the concept of spontaneity. Thermodynamics helps us answer whether or not a reaction will happen or if it needs an extra “push” to get started. For example, think about a ball rolling down a hill. You don't need to do anything to get it to roll down, but to roll it back up you need to put in energy to the system. Therefore, the first event is spontaneous, and the second event is nonspontaneous.
Some important terminology of this unit is the distinction between the system and the surroundings. In general, the system is where the “stuff” is happening, either a reaction or some thermodynamic process. The surroundings are everything outside of the system. Together, the system and surroundings make up the universe.
This unit will introduce two new measures of thermodynamics, entropy and Gibbs Free Energy, and connect them to this new idea of spontaneity. Enthalpy also makes an appearance in calculating values such as Gibbs Free Energy. You’ll learn new terms, including exergonic, endergonic, spontaneous, nonspontaneous, and many others, to explain the thermodynamic characteristics of a process.
Major Topics In Unit 9
9.1-9.2: Entropy
Entropy helps describe the measure of “disorder” in a system, essentially telling us the “orderliness” of a system. The more chaotic a system, the higher the entropy, and vice versa. Reactions can either increase the entropy of a system or decrease the entropy of a system. In the former case, the system becomes more disordered. In the latter, more ordered. Entropy is designated either by S°.
Entropy can also be calculated by using entropy values of certain elements, similar to the way enthalpy was calculated with enthalpies of formation. You’ll also learn about the laws of thermodynamics and observe how these laws dictate the way energy—both in the form of heat and entropy—acts in the natural world.
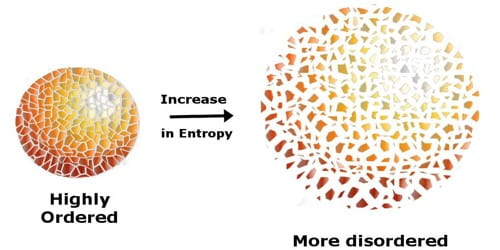
Image From AssignmentPoint
9.3-9.6: Gibbs Free Energy, Spontaneity, and Equilibrium
Together, entropy and enthalpy can be used to calculate the change in Gibbs Free Energy for a reaction. This value, notated as ΔG°, is a measure of spontaneity. Gibbs Free Energy tells us the amount of energy available to do work and is a function of entropy change and enthalpy change. If ΔG° is negative, the reaction is spontaneous, and if ΔG° is positive, the reaction is nonspontaneous. Another term we’ll learn that is synonymous with spontaneity are the terms thermodynamically favorable and thermodynamically unfavorable.
You’ll learn in 9.4 how kinetics connect to thermodynamics through a process known as kinetic control. This is when a reaction is limited by a high activation energy and so, despite being spontaneous, may not proceed at an observable rate. This means that kinetics can play a role in how spontaneous reactions act.
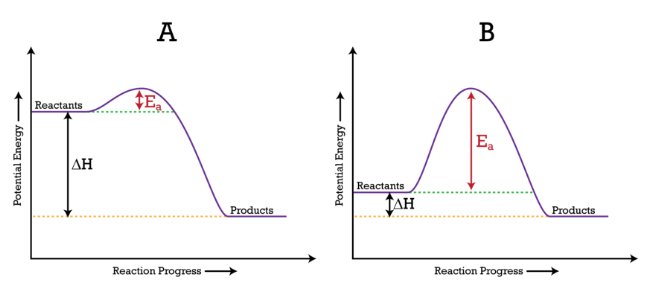
Image From Chemistry StackExchange
Spontaneity also has intimate connections to equilibrium. At nonstandard conditions, ΔG (note the lack of naught, meaning not at standard conditions) drives the reaction toward equilibrium and eventually reaches zero at the equilibrium point. ΔG can serve as an indicator as to whether a reaction is creating products, creating reactants, or is at equilibrium, similar to the way Q was used in Unit 7.
ΔG° also connects to the equilibrium constant K. A spontaneous reaction (one with a ΔG° < 0) will have a K value that is product favored or greater than 1. Similarly, a nonspontaneous reaction will have a K value that is reactant favored or less than 1. This helps us better understand the connection between equilibrium and thermodynamics.
Finally, coupled reactions will be used to make nonspontaneous processes spontaneous by combining reactions with common intermediates to form a mechanism for a new, spontaneous reaction.
9.7-9.10: Electrochemistry
The final topic in Unit 9 is electrochemistry. Electrochemistry is the thermodynamic study of redox reactions, reactions involving electron transfers. We can measure thermodynamic favorability in terms of cell potential. We’ll explore galvanic cells and electrolytic cells and how both connect to free energy changes, as well as Q and K.
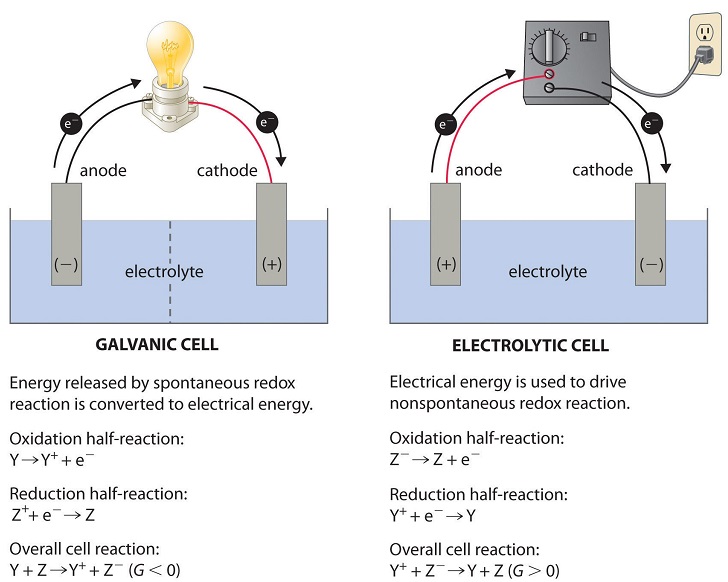
Image From LibreTexts

© 2024 Fiveable Inc. All rights reserved.