<< Hide Menu
Sumi Vora
Sumi Vora
Nitrogen Cycle 🌱
Nitrogen is a macro-nutrient, and the nitrogen cycle is the process by which it is exchanged between the atmosphere, land, and water. Nitrogen is an important component of proteins, DNA, and other biomolecules and is necessary for the growth and development of all living organisms.
The nitrogen cycle has a lot of chemical transformations, so while technically the exam can ask you about the nitrogen cycle, it tends to focus on later chapters and global trends, so don’t focus too much on memorizing all the chemical processes (this isn’t AP Chem!). Instead, focus on why the nitrogen cycle is important and what it can affect/how this change occurs and why.
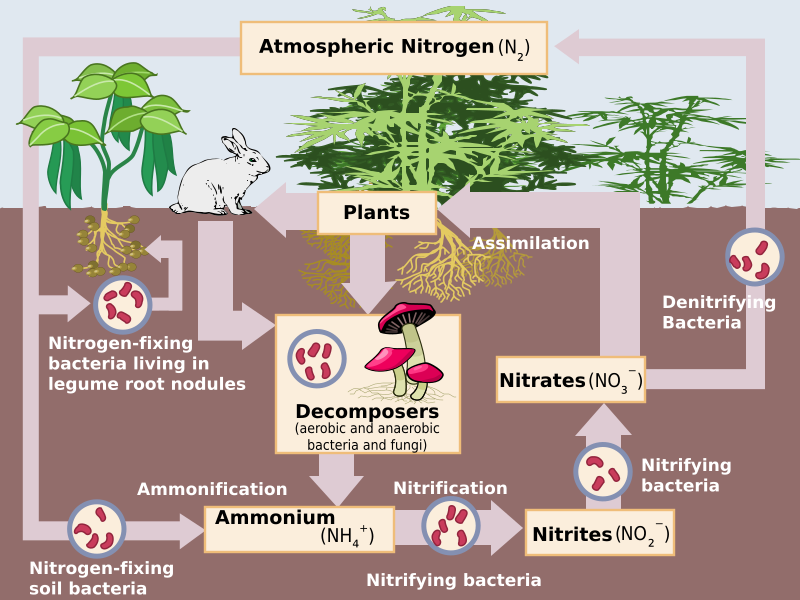
Image Courtesy of Wikimedia Commons
Nitrogen gas (N2) makes up 78% of the Earth's atmosphere, but it is not readily available to most living organisms. Nitrogen fixation allows nitrogen gas to be converted into a form that can be used by plants and animals, such as ammonia (NH3) or nitrate (NO3). Nitrogen fixation can happen in two different ways: biotic and abiotic. In the biotic pathway, nitrogen-fixing bacteria such as bacteria in the roots of certain plants convert nitrogen gas into ammonia (NH3), which then quickly bonds with hydrogen ions to become ammonium (NH4).
Later, during nitrification, ammonia and other compounds become nitrite (NO2) and later nitrate (NO3). Bacteria in soil and water allow this step of the nitrogen cycle to happen. It is important for bacteria to keep the amount of nitrate at bay to prevent pollution or overabundance of algal blooms in bodies of water. This can also occur in the atmosphere when N2 is exposed to lightning, fires, or fossil fuel combustion, which converts it directly to NO3. The nitrate then enters the soil through precipitation.
In assimilation, nitrogen is in its usable form, and gets absorbed by plant tissues. This nitrogen can be synthesized by consumers, or it can run into the ocean, providing aquatic ecosystems with enough nitrogen. When these organisms die, decomposition occurs and organic nitrogen present in these organisms through the nitrogen cycle reverts back into ammonium. This is called mineralization, and is the last step before nitrification can restart.
At the end of the nitrogen cycle, certain species of bacteria take nitrates and convert them into nitrous oxide (N2O) and eventually back into N2. This last step is appropriately called denitrification, and permits the cycle to once again restart.
- Nitrification: Nitrification is the process by which ammonia and other nitrogen compounds are converted into nitrite (NO2) and nitrate (NO3). Nitrification is carried out by bacteria in soil and water.
- Assimilation: Nitrate is taken up by plants through their roots and is incorporated into proteins, DNA, and other biomolecules.
- Denitrification: Denitrification is the process by which nitrate is converted back into nitrogen gas. Denitrification is carried out by bacteria in soil and water.
Once nitrogen is in usable form, plants can assimilate it, or incorporate it into their tissues. Consumers who eat the plants will also synthesize some of the nitrogen into their tissues. Some nitrogen also leaches into the ocean, either through runoff or precipitation. This is where aquatic organisms can obtain the nitrogen they need.
When organisms die, decomposers break down their tissues and convert the organic nitrogen (the nitrogen in their tissues) back into inorganic ammonium. This process is called mineralization or ammonification. After ammonification, the nitrification process can begin again.
The final step of the nitrogen cycle is denitrification, which returns nitrogen to the atmosphere. During denitrification, specialized bacteria convert nitrate into nitrous oxide (N2O) and then back into nitrogen gas (N2).
Human Impacts on the Nitrogen Cycle
Nitrogen is often classified as a limiting nutrient, meaning that its essentiality is coupled with the fact that it is scarce and hard to find. Since Earth is a closed system, there isn't a way for us to create more of it, but plants still require nitrogen to grow. As a result of this, nitrogen is common in fertilizers. While human usage of fertilizer now exceeds nature's fixation of it, its overuse can have negative consequences.
Even given nitrogen's ability to increase plant growth rate, excess nitrogen can quickly and severely disrupt balances in ecosystems of any size. In specific, an ecosystem's species richness, or the amount of species present, will die down due to the fact that plants with greater need for nitrogen will use it all and deplete it from plants with less need for it.
.

© 2024 Fiveable Inc. All rights reserved.