<< Hide Menu
2.7 Internal Energy and Energy Transfer
7 min read•june 18, 2024
Krish Gupta
Daniella Garcia-Loos
Krish Gupta
Daniella Garcia-Loos
This section gets to the heart of Thermodynamics. We will learn about the basic foundation on which the entire field of Thermodynamics is built upon: The Laws of Thermodynamics👓
In this section we will talk about two main ways to transfer energy.
- Work: which is probably very familiar to you from Physics 1 as the energy transferred due to a force acting over a distance. 📝
- Heat: energy transferred due to a difference in temperatures 🥵 This section is all about heat and work. We will look at processes where heat is added or removed or positive or negative work is done. We will look at the resulting changes in pressure, volume, temperature and energy. This is Thermodynamics at best.
The 0th law 🌟
That sound odd doesn't it? The Zeroth Law? Let’s take a look.
Two objects with different temperatures will result in a heat flow from the hotter object to the colder object until they reach the same temperature. The 0th law is built upon this idea.
The 0th law states if two objects are in equilibrium with a third object, then they are in equilibrium with each other.
Basically if you have a pencil, a pen and a marker, and the pencil is at the same temperature as the marker and the pen is at the same temperature as the marker, then the pen is at the same temperature as the pencil. That makes sense right? It’s basic logic. If two things are the same as the third then they are the same as each other.
Scientists made this assumption for a long time because it was simple logic. They did not feel that it had to be stated. That is why we know this as the 0th law because initially people just took it as a logical assumption.
Here are some key points about the 0th law of thermodynamics:
- The 0th law of thermodynamics is a fundamental principle that establishes a relationship between temperature and thermal equilibrium.
- It states that if two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
- In other words, the 0th law of thermodynamics states that if two systems are in thermal equilibrium, then they have the same temperature.
- The 0th law of thermodynamics is important because it allows us to define temperature and establish a scale for measuring it.
- It also allows us to understand how heat flows between different systems and how temperature affects the properties of matter.
- The 0th law of thermodynamics is a fundamental principle that underlies our understanding of heat and temperature, and is an important foundation for the study of thermodynamics.
The 1st Law 🌟
This is the law that you have probably heard since elementary school. It is foundational to many branches of science. This is also the law that is tested most often on the exam. With this law there is both a lot of theory and math associated so make sure to pay close attention.
The 1st Law states that heat energy is neither destroyed nor created in a thermodynamic system.
Here is the equation form for the 1st law which states that the change in internal energy is the sum of work and heat. Heat energy can be transferred but cannot be created or destroyed. This again is basically a restatement of the Conservation of Energy principle you learned last year. PHYSICS CONNECTS! Here are some key points about the first law of thermodynamics in bullet form:
- The first law of thermodynamics is a fundamental principle that describes the conservation of energy in a closed system.
- It states that energy cannot be created or destroyed, only converted from one form to another.
- In other words, the first law of thermodynamics states that the total energy of a closed system remains constant, regardless of the changes that take place within the system.
- The first law of thermodynamics can be expressed in the form of an equation, known as the energy conservation equation or the first law of thermodynamics equation. This equation is written as: ΔU = Q + W, where ΔU is the change in internal energy of the system, Q is the heat added to the system, and W is the work done by the system.
- The first law of thermodynamics is important because it allows us to understand how energy is converted and transferred between different forms and systems.
We learned about heat in the last section now let's learn about work.
Work 🔎
Work in thermodynamics is defined as the product of pressure and the change in volume. We will derive this equation soon.
Notice how both the starting and ending equations are the same. This is the derivation of how pressure times the change in volume is work. The negative sign is just there to remind you that if the gas expands negative work is done on the system because if the gas expands then the gas does the work not the surroundings. Therefore if the work done on the gas is negative if the gas expands and positive if the gas contracts.
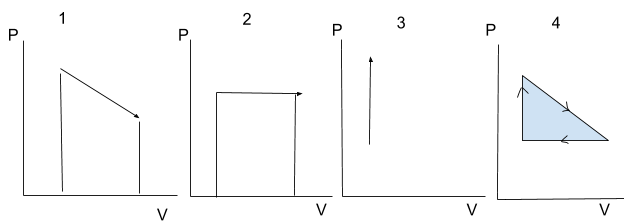
The work done by a process is equal to the area under it. The work done by a cycle is equal to the area of the shape.
Diagrams 1, 2 and 3 show individual processes. Note how in diagram 3 no work is done since there is no change in volume📕
Diagram 4 shows an entire cycle. You are much more likely to encounter this on the test.
Signs ✏️
Positive work is defined to be done on a system by the AP Physics 2 writers.
Note how in PV diagrams each process has an arrow. That helps us decide if the work done is positive or negative.
The gas does work when it expands as it has to exert pressure on the walls of the container to increase its volume.
For individual processes like ones shown in diagrams 1, 2 and 3, if the arrow points in the rightward direction work is negative and if the arrow points in the leftward direction work is positive.
For cycles like the one shown in diagram 4, if the arrows are clockwise then the work is negative and if they are counterclockwise, work is positive.
Be careful!!! All these sign conventions are from the standpoint of positive work being defined as work done ON the gas. If the AP exam tries to be tricky and asks about the work BY the gas then these signs would flip. However that is not very likely.
For heat it is much simpler. If heat is added to the gas then Q (heat) is positive and if heat is taken away from the gas then the Q (heat) is negative.
What is a PV diagram? 🧮
The 1st law of Thermodynamics in equation form can be represented as ΔU=W+Q
To solve Thermodynamic problems we have a very powerful tool called PV diagrams. PV diagrams are diagrams that show different processes and states of a gas. They tell us what is happening to the pressure and volume of the system.
The most important trait of the PV diagram is that the area under a process of a PV diagram will give us the work🤯
Now let’s look at the different processes we commonly see. These processes correlate to a line, curve or a stroke on the diagram.
- Isobaric - in this process the pressure of the gas is constant and only the volume changes. This process looks like a horizontal segment on a graph. - ΔU=W+Q
- Isochoric/Isovolumetric - in this process the volume of the container is held constant and the pressure is changed. This process looks like a vertical segment on a graph- ΔV=0- W=0- ΔU=Q
- Isotherm - in this process pressure increases and volume decreases or vice versa in ratios that the temperature is not changed. The temperature of the gas before and after is the exact same. - ΔU=0- Q=−W
- Adiabatic - This is the process in which no heat is exchanged. Volume is increased and pressure is decreased or vice versa in ratios where no heat is added to or taken from the system.
- Q=0
- ΔU=W Let's look at a diagram of some of these processes.
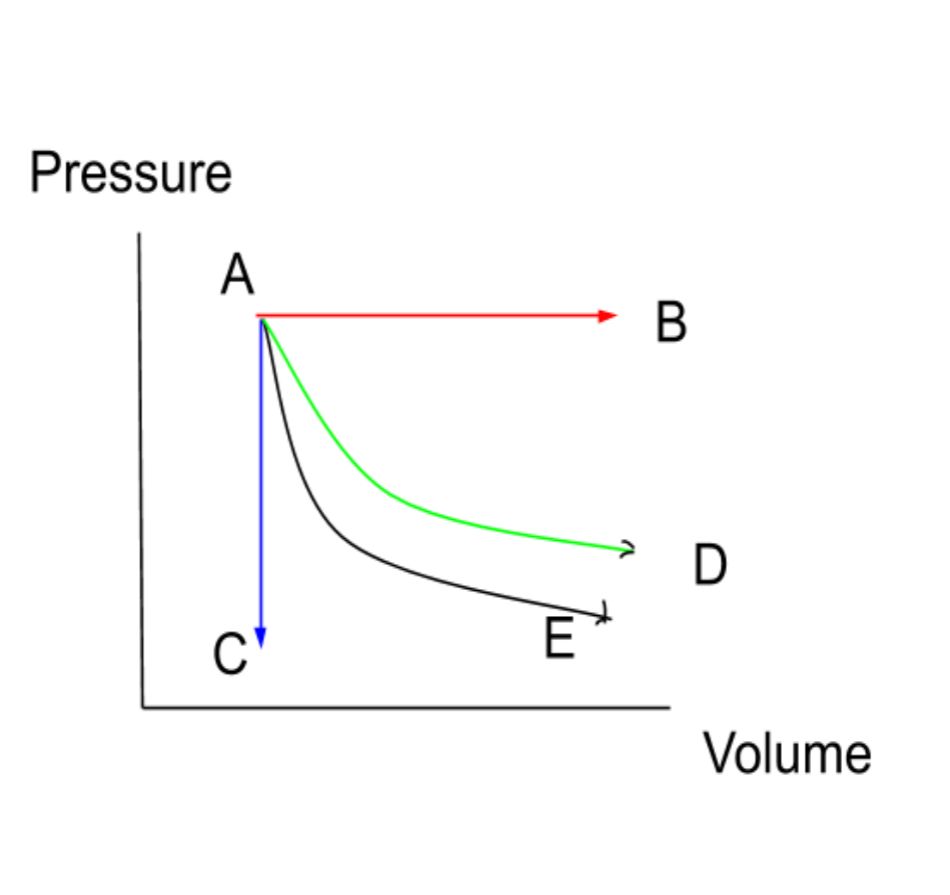
AB - Isobaric (horizontal line)
AC - Isochoric (vertical line)
AD - Isotherm (decreasing curve)
AE - Adiabatic (decreasing curve)
Here are steps for drawing a PV diagram:
- Determine the initial and final states of the system. The initial state is the starting point of the process, and the final state is the ending point.
- Choose an appropriate scale for the P-axis (pressure) and the V-axis (volume). The scale should be chosen such that the diagram fits on the page and the changes in pressure and volume are clearly visible.
- Draw the initial state as a point on the diagram. The coordinates of the point should be the initial pressure and volume of the system.
- Determine the path of the process. The path of the process is the path that the system follows on the PV diagram as it undergoes the process. The path can be straight or curved, depending on the process.
- Draw the path of the process on the PV diagram. Use a straight line for a constant-pressure process, a horizontal line for a constant-volume process, and a curve for any other process.
- Label the path with the type of process. For example, "isothermal expansion," "adiabatic expansion," etc.
- Draw the final state as a point on the diagram. The coordinates of the point should be the final pressure and volume of the system.
- Label the diagram with the initial and final states, as well as any other relevant information, such as the type of gas and the temperature.

© 2024 Fiveable Inc. All rights reserved.