<< Hide Menu
Peter Apps
Riya Patel
Peter Apps
Riya Patel
Overview
Electricity is everywhere! But what actually is electricity? In this unit we'll cover the basics of charge, and introduce the concepts of electrostatic force and electric potential difference. If you've taken AP Physics 1 (or another physics course before) some of these concepts may be familiar, but this is the foundation for the entire course, so don't skip it!
Big Ideas
- Force Interactions: Forces characterize interactions between objects or systems.
- Why does your hair stand on end after brushing it with a plastic comb?
- Fields: Fields predict and describe interactions.
- How does a charged rubber rod bend a stream of water?
- Conservation: Conservation laws constrain interactions.
- How are the kinematics of charged particles used in old televisions?
- Why is it sometimes necessary to shield against electric fields?
- How are maps of voltage and topographical maps related?
- Why can a bird land on a high voltage wire and not be electrocuted?
Exam Impact
Unit 1 will cover approximately 1/4 to 1/3 of the exam and should take around 40, 45-minute class periods to cover. The AP Classroom personal progress check has ~35 multiple choice questions and 1 free response question for you to practice on.
💡1.1: Electric Charge & Coulomb's Law
What is Charge? 🌩️
Charge is a fundamental property of subatomic particles. There are 2 types of charges: positive and negative. A proton has a positive charge, while an electron is negatively charged. The amount of charge on an object can be measured in either Coulombs (C) for large amounts of charge or elementary charges (e) for small amounts of charge. If you've taken chemistry already, you've most likely talked about the charge of atoms or ions as +1, -2, etc. These charges are measured in elementary charges. For this course, most of the time, we'll be dealing with "larger" amounts of charge and will use Coulombs as our primary unit of charge.
For virtually all of AP Physics C, we'll be talking about charges as point charges, which simply means that they are infinitely small objects with charge but no mass (meaning they take up no space).
Common Charges
| Name | Charge (Coulombs) | Charge (Elementary charge) |
| Proton | 1.6x10^-19 C | 1 e |
| Electron | -1.6x10^-19 C | -1 e |
| Neutron | 0 C | 0 e |
Law of Electrostatics
Simply put, "likes repel, opposites attract". 2 positive charges (+ and +) will repel each other, and likewise, 2 negative charges (- and -) also repel each other. Different charges (+ and -) be attracted to each other.
Try using the PhET simulation to see how a charged balloon can stick to the sweater as well as to the wall. While using it, try to visualize where the positive and negative point charges are interacting.
Attract or Repel?
| Name | Attracted to | Repelled by |
| Positive charge | Negative & Neutral | Positive |
| Negative charge | Positive & Neutral | Negative |
| Neutral object | Both Positive & Negative | Neither |
Conservation of Charge
When charged particles interact, the net amount of charge must remain constant.
In this example, a sphere with a +4e charge is touched to an identical sphere with a -12e charge, and then the two spheres are separated. Afterward, each sphere has a -4e charge. The -12e sphere transferred charge to the +4e sphere until they both reached -4e. The net charge of -8e was constant throughout this process.
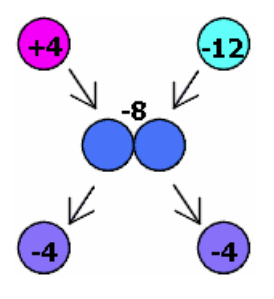
Image created by the author
Conductors and Insulators
The movement of charge in a substance depends on the the properties of the substance that the charge is trying to move through. In general there are 2 types of substances:
- Conductors: allow the charge to move easily through it.
- Insulators: restrict the movement of the charge.
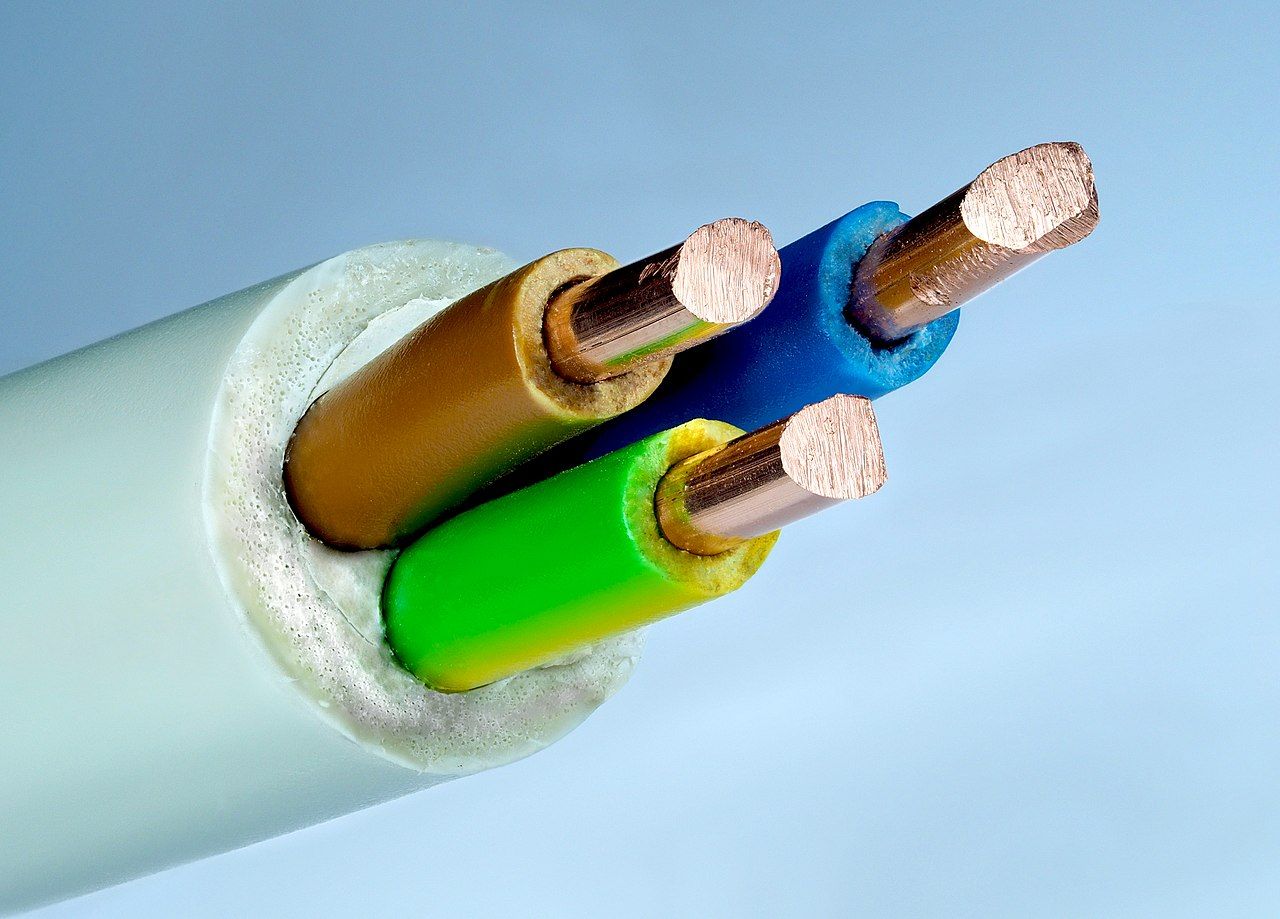
Image from Wikimedia
Often, we use insulators to prevent the charge from traveling, like in a typical household wire. The rubber insulator wraps the conductive copper wires to prevent short circuits or electrocution.
Charging and Discharging
There are 3 main ways to cause an object to become charged
- Friction: Rubbing two objects together can cause one object to lose electrons and the other to gain them. The object that loses electrons becomes positively charged, and the object that gains electrons becomes negatively charged.
- Contact: A charged object is touched to a neutral object. The neutral object gains the same charge as the initially charged object.
- Induction: A charged object is brought near but does not touch a neutral object. The neutral object becomes polarized. In this process, the opposite charge is attracted to the rod and moves closer, while the similar charge is repelled and moves farther away. If the neutral object is grounded, the similar charge will leave the sphere, which results in the sphere becoming oppositely charged in comparison to the original charged object. See below for an example.
Image from weebly.com
| Charging Method | Initial Charge | Contact? | Charge Movement | Final Charge |
| Friction/Rubbing | Both are neutral | Yes! More contact=More charge | Electrons move from the object with a weaker hold, to the object with a stronger hold | Two oppositely charged objects (one is +, the other is -) |
| Contact | One is neutral, one is charged | Brief Contact (or close proximity=sparks) | Electrons move between the objects until the charge is balanced on each | Both have the same charge |
| Induction (Temporary) | One is neutral, one is charged | No Contact | No grounding wire, like charges move away from the charged object, opposite charges move towards the charged object. Nothing leaves the neutral object. | Once the charged object leaves, the neutral object is still neutral. |
| Induction (Permanent) | One is neutral, one is charged | No Contact | With grounding wire, like charges leave the neutral object | Once the charged object leaves, the neutral object is left with a charge opposite to the original charged object. |
Electrostatic Force & Coulomb's Law
Coulomb's Law describes the force of attraction (or repulsion) experienced between two charged point objects. Point charges simply mean that we can approximate the charges as acting from a single point. The equation for calculating electrostatic force is given below:
where q1 and q2 represent the two charges, r is the distance between the charges, and ε (epsilon naught) is the Permittivity of Free Space constant given in your reference tables). Notice that if q1 and q2 are the same charge, we'll end up with a positive result. A positive electrostatic force value leads to repulsion between the two point charges.
** Physics Review Note: Electrostatic Force is a force! This means that we need to apply Newton's 3 Laws to the movement of charges too. One very common mistake is to forget that the two charged objects form a Newton's 3rd Law pair, the force between the two objects is equal in magnitude and opposite in direction.**
Let's take a look at some practice questions to test how well you understand the concepts that we've discussed in this guide so far!
Practice Questions:
1.

Answer:
Barun is correct. This is charging by induction. The positive rod coming close to the electroscope will cause the positive charge in the electroscope to be repelled, traveling down to the leaves which makes the leaves separate.
2.
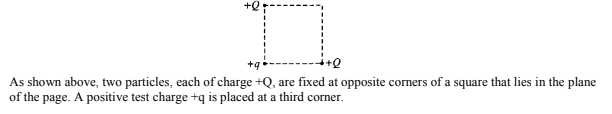
Image from collegeboard.org
a) What is the direction of the force on the test charge due to the two other charges?
b) If F is the magnitude of the force on the test charge due to only one of the particles, what is the net force acting on the test charge due to both of the charges?
Answers:
a) The net force must be directed towards the bottom left corner of the page. The test charge and both Q charges are positive so they must repel. The test charge is repelled downwards and to the left.
b) The test charge experiences two forces, both of magnitude F repelling it downwards and to the left. Using the Pythagorean theorem we can determine the resulting net force
3.
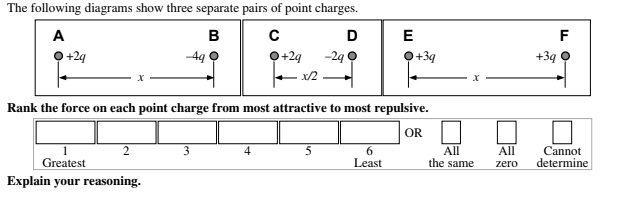
Image created by the author
Answer:
C = D > A = B > E = F
Each pair of point charges must have an equal force on them (look at the Newton's 3rd Law tip from earlier)! A&B and C&D have opposite charges so therefore, they must attract. E&F have the same charge and must be repelling. The magnitude of the force is directly proportional to the charges and inversely proportional to the square of the separation distance (💭 think back to the electrostatic force formula for the relationships here). Therefore, the shorter distance in C&D results in a larger force even though the charge in B has a greater magnitude.
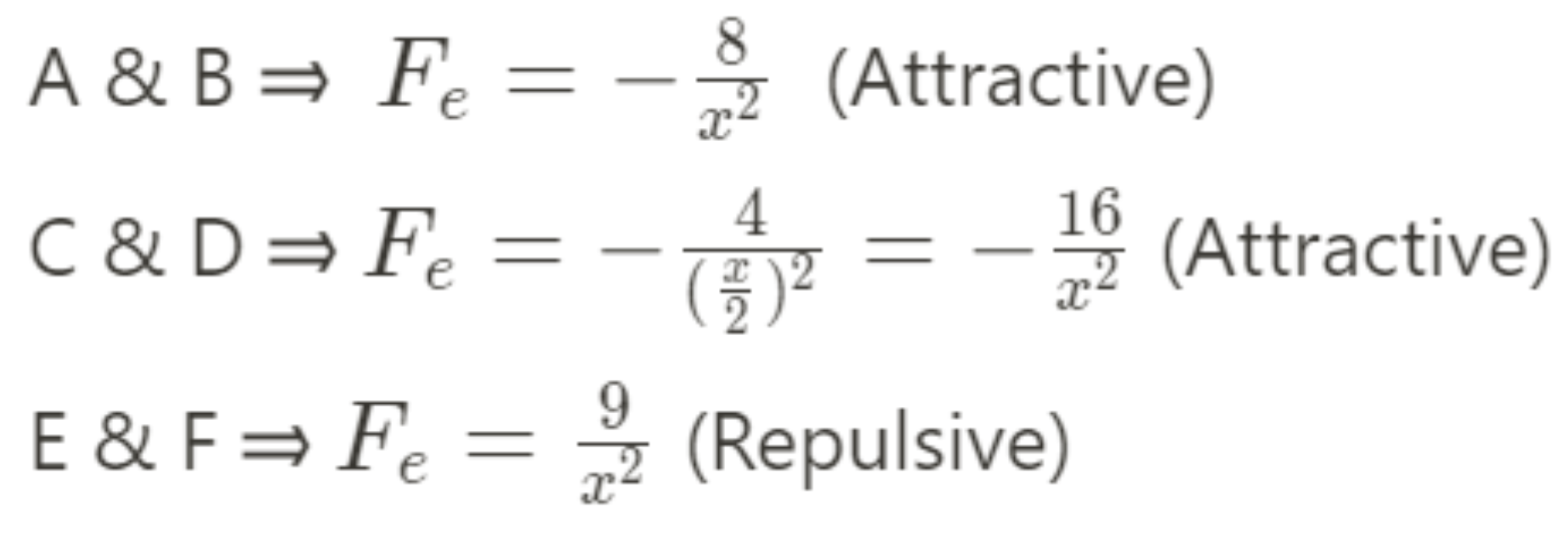

© 2024 Fiveable Inc. All rights reserved.